Structured Data Storage for Data-Driven Process Optimisation in Bioprinting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of a Round Robin Test
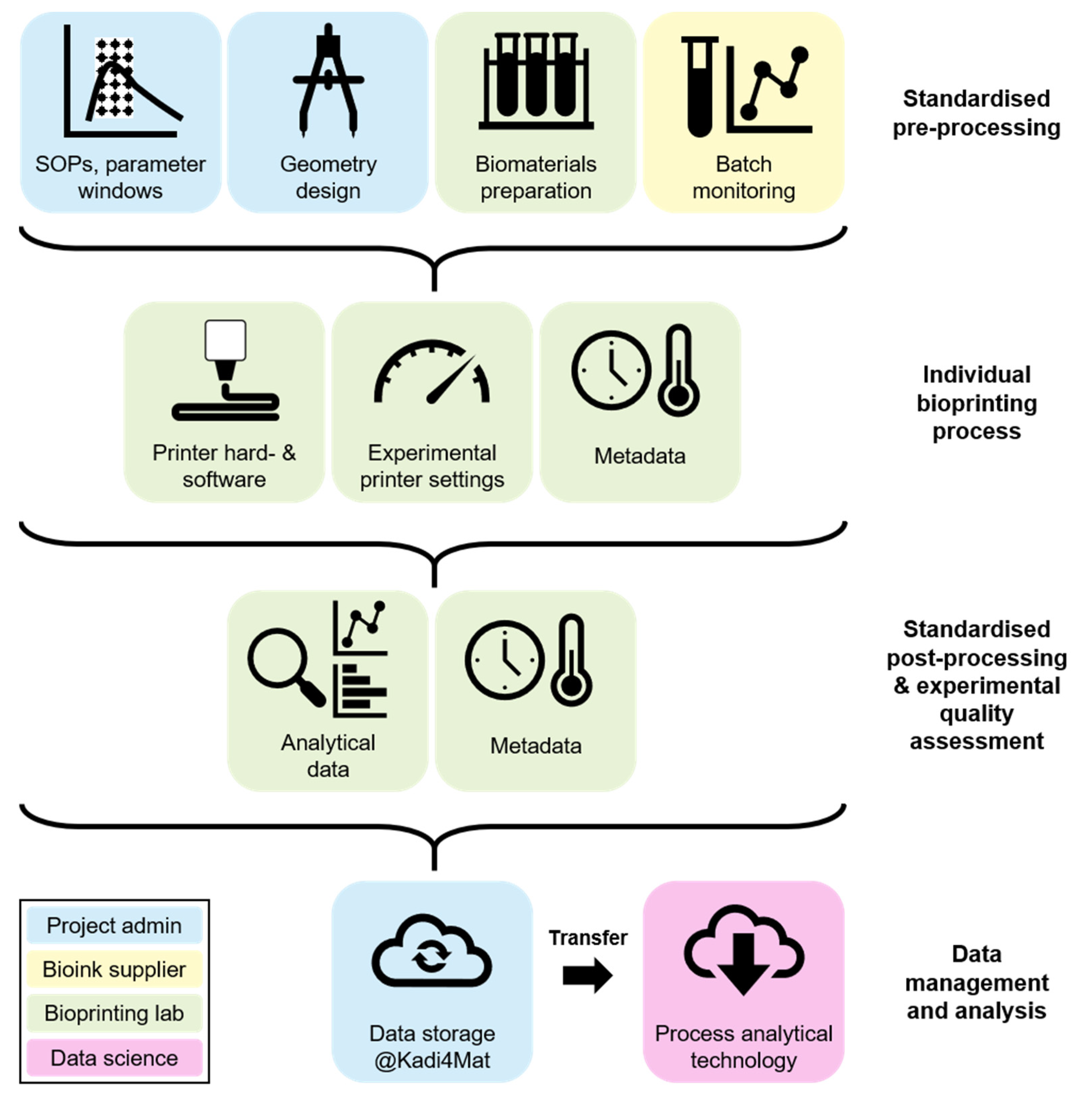
2.2. Design of Research Data Management
3. Results
3.1. Implementation of Round Robin Test
3.2. Data Management in Kadi4Mat
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, F.; Celli, J.; Rizvi, I.; Moon, S.; Hasan, T.; Demirci, U. A three-dimensional in vitro ovarian cancer coculture model using a high-throughput cell patterning platform. Biotechnol. J. 2011, 6, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, N.; Haddow, G.; Seymour, T.; Faulkner-Jones, A.; Shu, W. 3D bioprint me: A socioethical view of bioprinting human organs and tissues. J. Med. Ethics 2017, 43, 618–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilian, D.; Sembdner, P.; Bretschneider, H.; Ahlfeld, T.; Mika, L.; Lützner, J.; Holtzhausen, S.; Lode, A.; Stelzer, R.; Gelinsky, M. 3D printing of patient-specific implants for osteochondral defects: Workflow for an MRI-guided zonal design. Bio-Des. Manuf. 2021, 4, 818–832. [Google Scholar] [CrossRef]
- Mironov, V.; Boland, T.; Trusk, T.; Forgacs, G.; Markwald, R.R. Organ printing: Computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003, 21, 157–161. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [Green Version]
- Wenger, L.; Radtke, C.P.; Goepper, J.; Woerner, M.; Hubbuch, J. 3D-Printable and Enzymatically Active Composite Materials Based on Hydrogel-Filled High Internal Phase Emulsions. Front. Bioeng. Biotechnol. 2020, 8, 713. [Google Scholar] [CrossRef] [PubMed]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.A.; Groll, J.; Hutmacher, D.W. 25th Anniversary Article: Engineering Hydrogels for Biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef]
- Mancha Sánchez, E.; Gómez-Blanco, J.C.; López Nieto, E.; Casado, J.G.; Macías-García, A.; Díaz Díez, M.A.; Carrasco-Amador, J.P.; Torrejón Martín, D.; Sánchez-Margallo, F.M.; Pagador, J.B. Hydrogels for Bioprinting: A Systematic Review of Hydrogels Synthesis, Bioprinting Parameters, and Bioprinted Structures Behavior. Front. Bioeng. Biotechnol. 2020, 8, 776. [Google Scholar] [CrossRef]
- Pei, E.; Ressin, M.; Campbell, R.I.; Eynard, B.; Xiao, J. Investigating the impact of additive manufacturing data exchange standards for re-distributed manufacturing. Prog. Addit. Manuf. 2019, 4, 331–344. [Google Scholar] [CrossRef] [Green Version]
- Kawalkar, R.; Dubey, H.K.; Lokhande, S.P. A review for advancements in standardization for additive manufacturing. Mater. Today-Proc. 2021, 50, 1983–1990. [Google Scholar] [CrossRef]
- Li, P.; Faulkner, A.; Medcalf, N. 3D bioprinting in a 2D regulatory landscape: Gaps, uncertainties, and problems. Law Innov. Technol. 2020, 12, 1–29. [Google Scholar] [CrossRef]
- Di Prima, M.; Coburn, J.; Hwang, D.; Kelly, J.; Khairuzzaman, A.; Ricles, L. Additively manufactured medical products—The FDA perspective. 3D Print. Med. 2016, 2, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamroz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D Printing in Pharmaceutical and Medical Applications—Recent Achievements and Challenges. Pharm. Res. 2018, 35, 176. [Google Scholar] [CrossRef] [Green Version]
- Gillispie, G.; Prim, P.; Copus, J.; Fisher, J.; Mikos, A.G.; Yoo, J.J.; Atala, A.; Lee, S.J. Assessment methodologies for extrusion-based bioink printability. Biofabrication 2020, 12, 22003. [Google Scholar] [CrossRef]
- Shafiee, A.; Ghadiri, E.; Ramesh, H.; Kengla, C.; Kassis, J.; Calvert, P.; Williams, D.; Khademhosseini, A.; Narayan, R.; Forgacs, G.; et al. Physics of bioprinting. Appl. Phys. Rev. 2019, 6, 21315. [Google Scholar] [CrossRef]
- Rimann, M.; Bono, E.; Annaheim, H.; Bleisch, M.; Graf-Hausner, U. Standardized 3D Bioprinting of Soft Tissue Models with Human Primary Cells. JALA 2016, 21, 496–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunsberger, J.G.; Shupe, T.; Atala, A. An Industry-Driven Roadmap for Manufacturing in Regenerative Medicine. Stem Cells Transl. Med. 2018, 7, 564–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, J.; Chua, C.K.; Mironov, V. Application of Machine Learning in 3D Bioprinting: Focus on Development of Big Data and Digital Twin. Int. J. Bioprint. 2021, 7, 342. [Google Scholar] [CrossRef]
- Poessl, A.; Hartzke, D.; Schmidts, T.M.; Runkel, F.E.; Schlupp, P. A targeted rheological bioink development guideline and its systematic correlation with printing behavior. Biofabrication 2021, 13, 35021. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Labonte-Dupuis, T.; Demarquette, N.R.; Lerouge, S. A rheological approach to assess the printability of thermosensitive chitosan-based biomaterial inks. Biomed. Mater. 2021, 16, 15003. [Google Scholar] [CrossRef]
- Fisch, P.; Holub, M.; Zenobi-Wong, M. Improved accuracy and precision of bioprinting through progressive cavity pump-controlled extrusion. Biofabrication 2021, 13, 15012. [Google Scholar] [CrossRef]
- Strauss, S.; Schroth, B.; Hubbuch, J. Evaluation of the Reproducibility and Robustness of Extrusion-based Bioprinting Processes applying a Flow Sensor. Front. Bioeng. Biotechnol. 2022, 10, 831350. [Google Scholar] [CrossRef]
- Armstrong, A.A.; Alleyne, A.G.; Johnson, A.J.W. 1D and 2D error assessment and correction for extrusion-based bioprinting using process sensing and control strategies. Biofabrication 2020, 12, 45023. [Google Scholar] [CrossRef]
- Deneault, J.R.; Chang, J.; Myung, J.; Hooper, D.; Armstrong, A.; Pitt, M.; Maruyama, B. Toward autonomous additive manufacturing: Bayesian optimization on a 3D printer. MRS Bull. 2021, 46, 566–575. [Google Scholar] [CrossRef]
- Hourd, P.; Medcalf, N.; Segal, J.; Williams, D.J. A 3D bioprinting exemplar of the consequences of the regulatory requirements on customized processes. Regen. Med. 2015, 10, 863–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, H.; Mushnoori, S.; Higgins, B.; Kollipara, C.; Fermier, A.; Hausner, D.; Jha, S.; Singh, R.; Ierapetritou, M.; Ramachandran, R. A Systematic Framework for Data Management and Integration in a Continuous Pharmaceutical Manufacturing Processing Line. Processes 2018, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- Foster, E.D.; Whipple, E.C.; Rios, G.R. Implementing an institution-wide electronic lab notebook initiative. J. Med. Libr. Assoc. 2022, 110, 222–227. [Google Scholar] [CrossRef]
- Porr, M.; Marquard, D.; Stanislawski, N.; Austerjost, J.; Russo, M.; Bungers, S.; Klimmt, C.; Scheper, T.; Beutel, S.; Lindner, P. smartLAB—Working Interactively in a Digitalized Laboratory Environment. Chem. Ing. Tech. 2019, 91, 285–293. [Google Scholar] [CrossRef]
- Hanna, M.G.; Pantanowitz, L. The role of informatics in patient-centered care and personalized medicine. Cancer Cytopathol. 2017, 125, 494–501. [Google Scholar] [CrossRef] [Green Version]
- Higgins, S.G.; Nogiwa-Valdez, A.A.; Stevens, M.M. Considerations for implementing electronic laboratory notebooks in an academic research environment. Nat. Protoc. 2022, 17, 179–189. [Google Scholar] [CrossRef]
- re3data.org Project Consortium. Registry of Research Data Repositories. Available online: https://www.re3data.org/ (accessed on 14 June 2022).
- European Organization for Nuclear Research. OpenAIRE. Zenodo. Available online: https://www.zenodo.org/ (accessed on 14 June 2022).
- European Commission. Commission Welcomes Member States’ Declaration on EU Cloud Federation [Press Release]. Available online: https://digital-strategy.ec.europa.eu/en/news/commission-welcomes-member-states-declaration-eu-cloud-federation (accessed on 14 June 2022).
- European Commission. European Alliance for Industrial Data, Edge and Cloud. Available online: https://digital-strategy.ec.europa.eu/en/policies/cloud-alliance (accessed on 14 June 2022).
- Jain, A.; Persson, K.A.; Ceder, G. Research Update: The materials genome initiative: Data sharing and the impact of collaborative ab initio databases. APL Mater. 2016, 4, 53102. [Google Scholar] [CrossRef]
- Goodsell, D.S.; Zardecki, C.; Berman, H.M.; Burley, S.K. Insights from 20 years of the Molecule of the Month. Biochem. Mol. Biol. Educ. 2020, 48, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, R.H.; Anthofer, V.; Auer, S.; Baskaya, S.; Bischof, C.; Bronger, T.; Claus, F.; Cordes, F.; Demandt, É.; Eifert, T.; et al. NFDI4Ing—The National Research Data Infrastructure for Engineering Sciences. Available online: https://zenodo.org/record/4015201 (accessed on 14 June 2022).
- Scheffler, M.; Aeschlimann, M.; Albrecht, M.; Bereau, T.; Bungartz, H.-J.; Felser, C.; Greiner, M.; Groß, A.; Koch, C.T.; Kremer, K.; et al. FAIR data enabling new horizons for materials research. Nature 2022, 604, 635–642. [Google Scholar] [CrossRef]
- Brandt, N.; Griem, L.; Herrmann, C.; Schoof, E.; Tosato, G.; Zhao, Y.; Zschumme, P.; Selzer, M. Kadi4Mat: A Research Data Infrastructure for Materials Science. Data Sci. J. 2021, 20, 8. [Google Scholar] [CrossRef]
- Brandt, N.; Garabedian, N.T.; Schoof, E.; Schreiber, P.J.; Zschumme, P.; Greiner, C.; Selzer, M. Managing FAIR Tribological Data Using Kadi4Mat. Data 2022, 7, 15. [Google Scholar] [CrossRef]
- Garabedian, N.T.; Schreiber, P.J.; Brandt, N.; Zschumme, P.; Blatter, I.L.; Dollmann, A.; Haug, C.; Kümmel, D.; Li, Y.; Meyer, F.; et al. Generating FAIR Research Data in Experimental Tribology. Sci. Data 2022, 9, 315. [Google Scholar] [CrossRef]
- Draxl, C.; Scheffler, M. NOMAD: The FAIR concept for big data-driven materials science. MRS Bull. 2018, 43, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Tremouilhac, P.; Nguyen, A.; Huang, Y.-C.; Kotov, S.; Lütjohann, D.S.; Hübsch, F.; Jung, N.; Bräse, S. Chemotion ELN: An Open Source electronic lab notebook for chemists in academia. J. Cheminform. 2017, 9, 54. [Google Scholar] [CrossRef]
- Carpi, N.; Minges, A.; Piel, M. eLabFTW: An open source laboratory notebook for research labs. J. Open Source Softw. 2017, 2, 146. [Google Scholar] [CrossRef] [Green Version]
- Fielding, R.T. Architectural Styles and the Design of Network-Based Software Architectures. Ph.D. Dissertation, University of California, Irvine, CA, USA, 2000. [Google Scholar]
- Bernhardt, A.; Wehrl, M.; Paul, B.; Hochmuth, T.; Schumacher, M.; Schuetz, K.; Gelinsky, M. Improved Sterilization of Sensitive Biomaterials with Supercritical Carbon Dioxide at Low Temperature. PLoS ONE 2015, 10, e0129205. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Cauchois, G.; Schmitt, J.-F.; Louvet, N.; Six, J.-L.; Chen, Y.; Rahouadj, R.; Huselstein, C. Is there a cause-and-effect relationship between physicochemical properties and cell behavior of alginate-based hydrogel obtained after sterilization? J. Mech. Behav. Biomed. Mater. 2017, 68, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Lorson, T.; Ruopp, M.; Nadernezhad, A.; Eiber, J.; Vogel, U.; Jungst, T.; Luehmann, T. Sterilization Methods and Their Influence on Physicochemical Properties and Bioprinting of Alginate as a Bioink Component. ACS Omega 2020, 5, 6481–6486. [Google Scholar] [CrossRef]
- Rizwan, M.; Chan, S.W.; Comeau, P.A.; Willett, T.L.; Yim, E.K.F. Effect of sterilization treatment on mechanical properties, biodegradation, bioactivity and printability of GelMA hydrogels. Biomed. Mater. 2020, 15, 65017. [Google Scholar] [CrossRef] [PubMed]
- Stoppel, W.L.; White, J.C.; Horava, S.D.; Henry, A.C.; Roberts, S.C.; Bhatia, S.R. Terminal sterilization of alginate hydrogels: Efficacy and impact on mechanical properties. J. Biomed. Mater. Res. Appl. Biomater. 2014, 102, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and Shape Fidelity of Bioinks in 3D Bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef] [PubMed]
- Diamantides, N.; Dugopolski, C.; Blahut, E.; Kennedy, S.; Bonassar, L.J. High density cell seeding affects the rheology and printability of collagen bioinks. Biofabrication 2019, 11, 45016. [Google Scholar] [CrossRef]
- Zidan, A.; Alayoubi, A.; Asfari, S.; Coburn, J.; Ghammraoui, B.; Aqueel, S.; Cruz, C.N.; Ashraf, M. Development of mechanistic models to identify critical formulation and process variables of pastes for 3D printing of modified release tablets. Int. J. Pharm. 2019, 555, 109–123. [Google Scholar] [CrossRef]
- Šljivic, M.; Pavlovic, A.; Kraišnik, M.; Ilić, J. Comparing the accuracy of 3D slicer software in printed enduse parts. IOP Conf. Ser. Mater. Sci. Eng. 2019, 659, 012082. [Google Scholar] [CrossRef]
- Prendergast, M.E.; Burdick, J.A. Recent Advances in Enabling Technologies in 3D Printing for Precision Medicine. Adv. Mater. 2020, 32, e1902516. [Google Scholar] [CrossRef]
- Schmieg, B.; Gretzinger, S.; Schuhmann, S.; Guthausen, G.; Hubbuch, J. Magnetic Resonance Imaging as a tool for quality control in extrusion-based bioprinting. Biotechnol. J. 2022, 17, e2100336. [Google Scholar] [CrossRef]
- Chimene, D.; Kaunas, R.; Gaharwar, A.K. Hydrogel Bioink Reinforcement for Additive Manufacturing: A Focused Review of Emerging Strategies. Adv. Mater. 2020, 32, 1902026. [Google Scholar] [CrossRef]
- Gomez-Blanco, C.J.; Mancha-Sanchez, E.; Marcos, A.C.; Matamoros, M.; Diaz-Parralejo, A.; Blas Pagador, J. Bioink Temperature Influence on Shear Stress, Pressure and Velocity Using Computational Simulation. Processes 2020, 8, 865. [Google Scholar] [CrossRef]
- Gao, T.; Gillispie, G.J.; Copus, J.S.; Kumar, P.R.A.; Seol, Y.J.; Atala, A.; Yoo, J.J.; Lee, S.J. Optimization of gelatin-alginate composite bioink printability using rheological parameters: A systematic approach. Biofabrication 2018, 10, 34106. [Google Scholar] [CrossRef]
- Strauß, S.; Meutelet, R.; Radosevic, L.; Gretzinger, S.; Hubbuch, J. Image analysis as PAT-Tool for use in extrusion-based bioprinting. Bioprinting 2021, 21, e00112. [Google Scholar] [CrossRef]
- Matamoros, M.; Gómez-Blanco, J.C.; Sánchez, Á.J.; Mancha, E.; Marcos, A.C.; Carrasco-Amador, J.P.; Pagador, J.B. Temperature and Humidity PID Controller for a Bioprinter Atmospheric Enclosure System. Micromachines 2020, 11, 999. [Google Scholar] [CrossRef]
- Shen, Z.; Shang, X.; Zhao, M.; Dong, X.; Xiong, G.; Wang, F.-Y. A Learning-Based Framework for Error Compensation in 3D Printing. IEEE Trans. Cybern. 2019, 49, 4042–4050. [Google Scholar] [CrossRef]
- Gretzinger, S.; Beckert, N.; Gleadall, A.; Lee-Thedieck, C.; Hubbuch, J. 3D bioprinting—Flow cytometry as analytical strategy for 3D cell structures. Bioprinting 2018, 11, e00023. [Google Scholar] [CrossRef]
- Schmieg, B.; Nguyen, M.; Franzreb, M. Simulative Minimization of Mass Transfer Limitations Within Hydrogel-Based 3D-Printed Enzyme Carriers. Front. Bioeng. Biotechnol. 2020, 8, 365. [Google Scholar] [CrossRef]
- Kadi4Mat Team and Contributors. IAM-CMS/kadi-apy: Kadi4Mat API Library (0.21.0). Available online: https://zenodo.org/record/6623518 (accessed on 14 June 2022).
- Kadi4Mat Team and Contributors. IAM-CMS/kadi: Kadi4Mat (kadi-v0.25.1). Available online: https://zenodo.org/record/6623521 (accessed on 14 June 2022).



| Process Parameter/ Consumable/ Device | Possible Findings | Impact of Deviation | Standardised Parameter within Scope of Experiments |
|---|---|---|---|
| Bioink raw material | Batch-to-batch variations, ageing, change of composition by purification or sterilisation steps [46,47,48,49,50] | Variations in viscosity, rheological behaviour and printing properties [14,20,51,52]; risk of process interruption because of nozzle clogging or increased bioink flow | Batch monitoring: analysis protocols for physicochemical characterisation, rheology, degree of (individual) functionalisation (adapted to individual bioink) |
| Components are used as received at bioprinting lab | |||
| SOP for storage | |||
| Bioink preparation | Commonly still a manual step [14]. May lead to inhomogeneities, air bubbles [53], etc. | Deviations in bioink flow within one experimental run or between runs [22,53] | SOPs for preparation steps, standardised consumables, documentation of deviations |
| Images of cartridge for visual air bubble control | |||
| Small batches (limit storage time of preparations within process) | |||
| Geometry transfer from design to local device | Adaption to local software and printer [25,54]; individual settings (user-controlled and algorithm-based) | Different printing outcome | Pre-defined design of basic geometries (line, circle, edges) that are possible with all hardware equipment |
| Use of small object sizes for high number of technical replicates | |||
| SOPs for parameter window of user-controlled settings | |||
| Documentation of algorithm-based deviations by user | |||
| Printer hardware and software | Resolution of printers [55], position effects on printing platform, availability of settings/addons such as temperature control jackets, flow settings [16,21,56] | Different printing outcome [57,58] | Transfer by user according to process window of SOP |
| Lack of process control for simple devices without addons | Documentation and characterisation of used addons and parameters | ||
| Experimental extrusion parameters device /software | Deviations in bioink flow [22], response time and acceleration of device at start/end of movement | Experimental geometry deviations: closed/open circles, line uniformity and thickness [23,59,60] | Operate within pre-defined process window, document parameters and collect comments of user |
| Non-biological consumables for printing | Cartridge size is dependent on hardware. Possibility of dead-volume effects and acceleration | Surface tension effects on filament extrusion | Standardised material of single-use components as cartridges. Standardised transferable items (nozzles and wellplates) |
| Document type of consumable used | |||
| Ambient conditions | Temperature, humidity, process duration [58,61,62] | Rheology deviations, bioink ageing, drying of recently printed scaffold part | Set parameter window, set max process time, document experimental values |
| Resulting geometry–imaging | Drying of samples [60], reflective surfaces, low contrast | Individual image quality | Standardized devices, pre-set imaging parameters, scale bar for image analysis |
| Biological functionality (highly dependent on individual application) | Deviations in biological functionality (cell viability [63], diffusion limitations [64]) compared to expected values or control group | Decreased reproducibility of assays | Analysis of results only with consideration of experimental conditions of the whole process |
| Decreased biological functionality as a result of other process deviations |
| Template | Type of Data | Allocated Information, Datasets and/or Files |
|---|---|---|
| Standardised bioink preparation | Fixed metadata | Changelog |
| Description for user: Aim of template and instructions on how to apply it (includes embedded images) | ||
| Link to: corresponding SOPs | ||
| Generic metadata | Experiment identification number | |
| Name and batch identifier of bioink | ||
| User identification (anonymised) | ||
| Timestamps of preparation and storage duration | ||
| Weighed portions of bioink components | ||
| Checkpoints: Bioink preparation executed as specified in SOP? | ||
| Deviations/comments (free text option for user) | ||
| Attached files (to generated record) | Image on ready-to-use bioink cartridge before bioprinting for air bubble assessment | |
| Linked records (to generated record) | Raw material analysis | |
| Individual bioprinting process | ||
| Individual bioprinting process (within pre-defined window of operation) | Fixed metadata | Analogous to template “Standardised bioink preparation” |
| Generic metadata | Experiment identification | |
| User identification (anonymised) | ||
| Timestamp of start and end | ||
| Temperature (ambient conditions, 3D printer cabinet, heating mantle, nozzle heater) | ||
| Printer settings (flowrate, printhead speed, layer height, pre-/postflow, tear off settings at end of strands, etc.) | ||
| Checkpoints: used consumables | ||
| Deviations/comments | ||
| Attached files (to generated record) | Bioprinting log files, images, comment files of experimental deviations | |
| Linked records (to generated record) | Used bioink preparation | |
| Used hardware and methods | ||
| Description of used hardware or method | Fixed metadata | Analogous to template “Standardised bioink preparation” |
| Generic metadata | Identification of method/device (supplier, version) | |
| User identification (anonymised) | ||
| Description of connected process steps and hardware addons (example bioprinter: manufacturer, model, configuration of device, type of air flow in printer cabinet, software, used calibration method, etc.) | ||
| Attached files (to generated record) | Individual data files, image of hardware for visualisation | |
| Linked records (to generated record) | (links from individual bioprinting process are incoming) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmieg, B.; Brandt, N.; Schnepp, V.J.; Radosevic, L.; Gretzinger, S.; Selzer, M.; Hubbuch, J. Structured Data Storage for Data-Driven Process Optimisation in Bioprinting. Appl. Sci. 2022, 12, 7728. https://doi.org/10.3390/app12157728
Schmieg B, Brandt N, Schnepp VJ, Radosevic L, Gretzinger S, Selzer M, Hubbuch J. Structured Data Storage for Data-Driven Process Optimisation in Bioprinting. Applied Sciences. 2022; 12(15):7728. https://doi.org/10.3390/app12157728
Chicago/Turabian StyleSchmieg, Barbara, Nico Brandt, Vera J. Schnepp, Luka Radosevic, Sarah Gretzinger, Michael Selzer, and Jürgen Hubbuch. 2022. "Structured Data Storage for Data-Driven Process Optimisation in Bioprinting" Applied Sciences 12, no. 15: 7728. https://doi.org/10.3390/app12157728






